Cytosponge test for patients with chronic gastro-oesophageal reflux disease (GORD)
Background
- Heartburn/regurgitation symptoms caused by GORD affect up to 20% of adults leading to significant annual healthcare costs. Most do not have a diagnosis and are treated over many years with acid-suppressant medication therapy. Symptoms of heartburn are important when one considers the link between heartburn and oesophageal adenocarcinoma (OAC).
- It is estimated that 3–6% of individuals with GOR-predominant symptoms could have the precursor lesion to oesophageal adenocarcinoma, Barrett’s oesophagus. However, only around 20% of patients with Barrett’s oesophagus are diagnosed or known about. Therefore, most cases of oesophageal adenocarcinoma are diagnosed de novo, without the opportunity to prevent Barrett’s progression through surveillance.
- The incidence of oesophageal adenocarcinoma is six times higher than it was in the 1990s. Oesophageal adenocarcinoma also has a poor prognosis due to late presentation, with an overall 5-year survival of less than 20%, despite advances in neoadjuvant therapy and surgery.
- Treatment of dysplastic Barrett’s oesophagus prevents progression to adenocarcinoma; however, the optimal diagnostic strategy for Barrett’s oesophagus is unclear.
- Clinical guidelines recommend urgent referral for an endoscopy in patients with warning symptoms, such as dysphagia and weight loss, and routine referral for an endoscopy in those with symptoms of gastro-oesophageal reflux that persist despite recommended lifestyle and pharmacological management strategies, and those with multiple additional risk factors for the disease.
- Modelling using data from the USA estimated that the burden of oesophageal adenocarcinoma could be reduced by up to 50% through implementing strategies for the systematic screening and early diagnosis of Barrett’s in individuals with gastro-oesophageal reflux, who would otherwise not have been referred for an endoscopy.
- Early detection needs to be combined with effective interventions to be clinically beneficial. There have been important advances in outpatient-based endoscopic therapies, which are now recommended for low-grade and high-grade dysplasia in Barrett’s oesophagus, with high rates of success (approx. 90%) and low rates of recurrence.
- Patients with intramucosal stage I cancers have a survival of more than 90% and can be treated endoscopically, thus mitigating the risks of and side-effects from systemic therapy and an oesophagectomy, which is often required for more advanced disease.
- The Cytosponge-trefoil factor 3 (TFF3) test (“Cytosponge”) is a non-endoscopic test for Barrett’s oesophagus – see section 3 (below) for details of the procedure.
- Scottish government has approved and funded the roll out of Cytosponge as an alternative investigative modality to upper GI endoscopy for two groups of patients, the inclusion criteria being:
- Patients with reflux-predominant upper GI symptoms, who have been referred for upper GI endoscopy with concern of Barrett’s oesophagus, pre- or early oesophageal cancer
- Patients with known Barrett’s oesophagus who are at risk of oesophageal cancer with progressive symptoms and/or who have had significant delay in their upper GI endoscopy.
1. Cytosponge in NHS Lothian
- Cytosponge clinics are now established as an alternative to upper GI endoscopy for Barrett’s surveillance. Over 500 patients (as of September 2021) have undergone Cytosponge procedures successfully and safely, with encouraging results.
- We are now gearing up to roll this out for patients referred with symptoms that are likely to be the result of chronic reflux and who are therefore at risk of harbouring Barrett’s. This is the key finding in patients with chronic reflux symptoms as:
(a) when OG cancer presents with symptoms these are invariably ‘alarm’ or ‘red flag’ in nature (when a careful history is taken) and not those of simple reflux/dyspepsia
(b) early oesophageal adenocarcinoma(OAC) or dysplasia is usually a chance finding, albeit important, not the cause of the symptoms; and will be detected by Cytosponge.
(c) early squamous cell carcinoma (SCC) is not well detected by Cytosponge, but does not present with reflux symptoms. It is less common than OAC and strategies for early detection are lacking.
(d) most other findings will be treated symptomatically – hiatus hernia, different grades of oesophagitis, etc. Those with grade C oesophagitis no longer need repeat endoscopy to check mucosal healing and those harbouring Barrett’s will be detected by Cytosponge. Grade D oesophagitis will either respond to medical therapy or, if not, will lead to re-referral or a stricture, either should then trigger an upper GI endoscopy.
- Cytosponge will only be of clinical benefit when performed in patients with GORD and it can be difficult to tell who these might be from symptoms or history: typical heartburn and regurgitation are specific for GORD when they predominate but this only occurs in 50% of proven refluxers and other symptoms e.g. chest/epigastric pain, nausea, cough etc. are common in GORD. Cytosponge is of no value if performed in patients with e.g. Helicobacter-related conditions, functional dyspepsia, IBS, biliary colic etc.
- It is not possible to provide hard and fast referral guidelines but the following principles should be followed:
- Patient with typical reflux-predominant symptoms-heartburn/regurgitation/belching/waterbrash (not ‘atypical’ symptoms e.g. unexplained cough/undiagnosed chest pain) and not patients with epigastric pain, bloating, satiety etc.
- The GERD-Q questionnaire is a simple, validated scoring system that can be used to score patients as likely to suffering from reflux or not. A score of 8+ is indicative of GORD. It would be beneficial to have this when patients are referred but, if not, it will be performed during telephone pre-assessment when referrals are vetted. Those with a low score will be returned to the GI Consultant who triaged the original referral for alternative management and/or clinic review.
https://fpnotebook.com/gi/Exam/GrdqQstnr.htm

- Age 50y or older (Barrett’s is rare before this age). Cytosponge can be considered in younger patients with additional risk factors e.g. obesity, long duration symptoms, family history of Barrett’s or oesophageal cancer.
- Chronic symptoms (years); not young patients with a short history of reflux or who have not been through all the measures usually advised for management of reflux.
- Scottish Government guidance includes the use of Cytosponge for patients referred with dysphagia and a low Edinburgh Dysphagia Score (EDS <3.5) and some Boards have adopted this. For the time-being, we are no not doing this – dysphagia referrals should still be referred using established referral guidelines.
- In addition, there are nationally agreed exclusion criteria for Cytosponge, as follows:
- Pregnancy
- Significant dysphagia or swallowing disorders
- Suspected or known chronic liver disease/cirrhosis
- Oesophageal or gastric varices
- Previous or Current Oesophageal Tumour
- Recent invasive oesophageal procedure within the past 2 months e.g. dilatation, endoscopic mucosal resection (EMR), radiofrequency ablation (RFA) etc.
- Previous oesophageal surgery including fundoplication
- Patients in whom results would not influence further management as a result of severe co-morbidities or general fitness
- Inability to give consent due to lack of capacity
2. Cytosponge procedure in brief
- The Cytosponge test is performed in an outpatient setting e.g. clinic room.
- It is classified as a non-aerosol-generating procedure (AGP).
- Sedation is not required.
- Fasting patients are asked to swallow a polyethylene sponge constrained inside a gelatine capsule, attached to a string.
- Once in the stomach the capsule dissolves in 7 minutes, allowing sponge to expand.
- It is then withdrawn up the oesophagus and retrieved by the person administering the procedure.
- The sponge, containing exfoliated cells from the oesophagus, is placed in fixative and sent for laboratory analysis – cytological assessment and staining for expression of TFF-3 (an accurate marker of intestinal metaplasia, a key feature of Barrett’s) and p53 expression (a key marker of dysplasia).
The entire procedure episode takes 10-15 minutes.
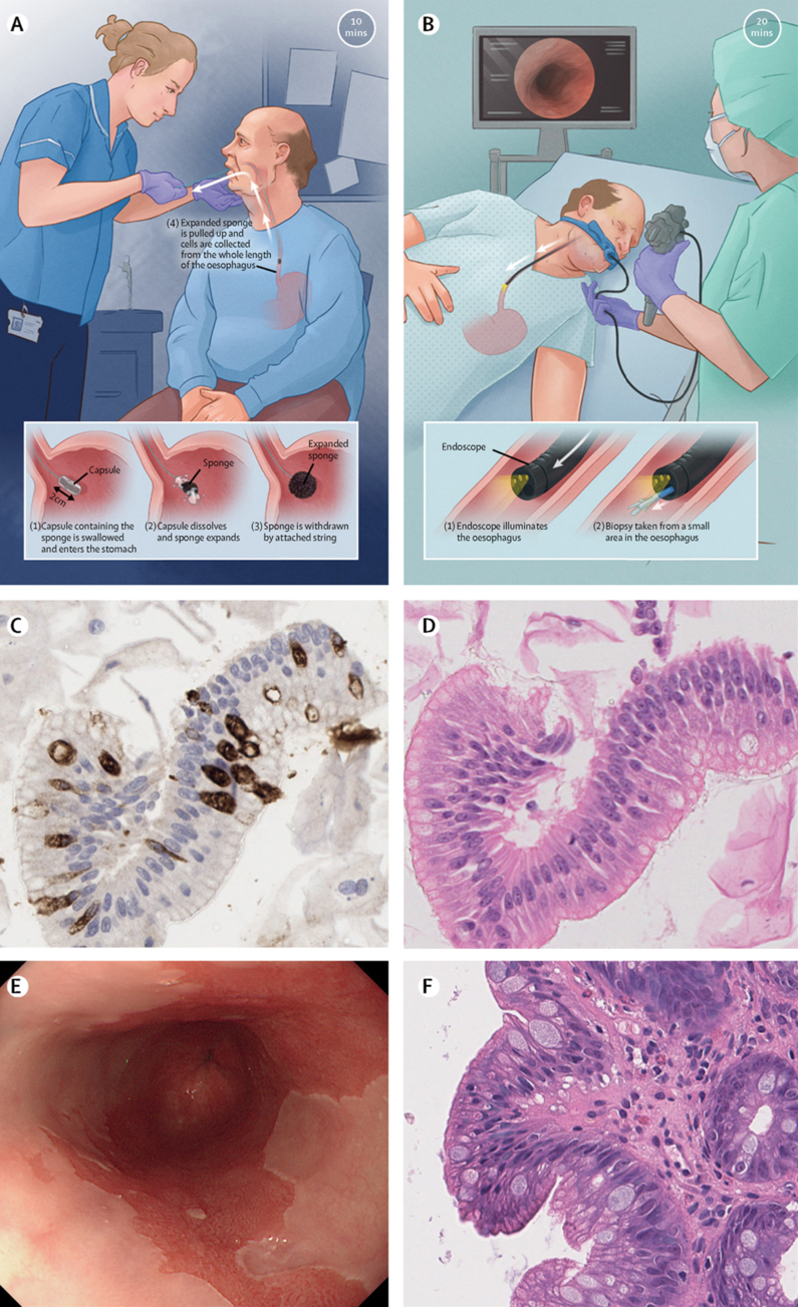
Cytosponge (panels A,C,E) compared to standard upper GI endoscopy (panels B,D,F). from Fitzgerald RC et al Lancet 2020;396 (10247):333-344.
https://doi.org/10.1016/S0140-6736(20)31099-0
3. Cytosponge Process
- For Primary Care – refer via SCI Gateway (RIE >> Gastroenterology – Medical >> LI GI – Upper). Providing the GERD-Q score with the referral (or the free text information that would allow secondary care to calculate the score easily would enhance referrals).
- For secondary Care – in TRAK, select ‘Cytosponge New’ as the TRAK outcome in the drop-down list of triage options.
- GI Admin team will collate referrals alongside the cohort of Barrett’s surveillance patients.
- Referrals undergo telephone pre-assessment to discuss procedure, assess suitability and complete paperwork including GERD-Q.
- Those felt not be suitable will be returned to the GI team in secondary care for alternative investigation/clinic appointment.
- Rest will be booked for Cytosponge at SJH, WGH, RIE or LCTC.
- Symptoms checked at time of procedure to ensure no upper GI ‘alarm’ symptoms present. Procedure also affords opportunity for brief intervention re management of chronic reflux (diet, lifestyle, weight loss etc.) and provision of patient information.
- Procedure performed and samples sent to pathology for analysis.
- Results from accredited central reporting lab (‘Cyted’, Cambridge) are returned to a central mailbox, collated and summarised on a database.
- Proforma template results letters have been developed. At present the clinical team involved in Cytosoponge will dictate the results letters to the patient, GP and referring clinician, completing the episode. In future, as the system becomes familiar and embedded, this may be devolved back to referring clinicians.
- Patients with results reporting ‘atypia’, aberrant p53 expression or dysplasia will undergo urgent endoscopy. Those with results consistent with having Barrett’s will undergo routine endoscopy while those with negative results will be managed as for GORD.
- Data is audited at regular intervals.
Ian Penman, Consultant Gastroenterologist
NHS Lothian
October 2021













