Recommendations for Management of DMARDs and Biologics in the Peri-Operative Period
Evidence for the management of DMARDs and Biologics in the peri-operative period is limited but national and international guidelines do exist1,2,3.
It has been agreed by NHS Lothian Rheumatic Diseases unit that there will be local adoption of the British Society for Rheumatology (BSR) Guidelines on safety of biologics (2019) and prescribing & monitoring of DMARDs (2017) to advise on management of these medicines in the peri-operative period. This covers all surgical procedures including dental surgery.
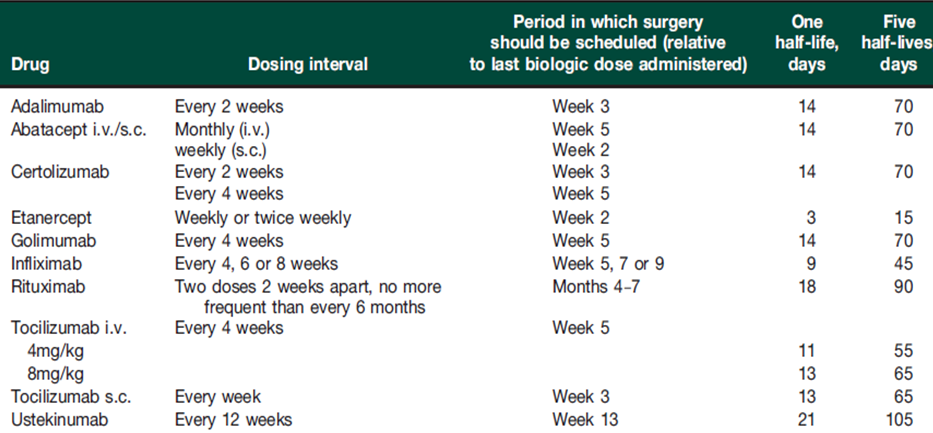
In general the advice is to withhold biologic for 1 dosing interval before elective surgery, except for tocilizumab which has a significant effect on acute phase response and should be withheld for 2 intervals.
This has been extrapolated for biologic agents not included in the BSR guideline.
References
- Holroyd C et al. The British Society for Rheumatology biologic DMARD safety guidelines in inflammatory arthritis. Rheumatology (2019) 58(2) 220–226.
- Ledingham J et al on behalf of the BSR and BHPR Standards, Guidelines and Audit Working Group, BSR and BHPR guideline for the prescription and monitoring of non-biologic disease-modifying anti-rheumatic drugs. Rheumatology (2017) 56(6) 865–868.
- American College of Rheumatology/American Association of Hip and Knee Surgeons Guideline for the Perioperative Management of Antirheumatic Medication in Patients With Rheumatic Diseases Undergoing Elective Total Hip or Total Knee Arthroplasty. Arthritis Care & Research (2017) 69 (8) 1111–1124.
M.A & H.B/S.R 25-01-24













