In February 2021, the Chief Medical Officers of the UK recommended supplementation with 400 units of vitamin D daily to those over the age of 65 years, and to people who are not exposed to much sun. There was no mention of investigation or monitoring. There is Scottish Government advice on Vitamin D for all ages, with a link to a patient information leaflet. The Vitamin D patient information leaflet is also available in multiple other languages.
This guideline applies to symptomatic adults and/or those with abnormal biochemistry requiring investigation, diagnosis and treatment. It also highlights those with medical conditions which may put them at risk of vitamin D deficiency, or cause treatment with vitamin D to be complex-. It should be used where a patient’s clinical features warrant prior assessment relating to their Vitamin D status or where investigation or monitoring may be indicated.
Examples include:
- symptoms which may indicate the need for high dose treatment with vitamin D
- risk of complications from treatment with vitamin D due to a hypercalcaemic disorder, previous renal stones, renal impairment or granulomatous disorder.
This guideline does not apply to asymptomatic people with risk factors for Vitamin D deficiency, who should be given lifestyle advice and advised to take a standard supplement.
There is a separate set of Vitamin D guidelines for children and young people on the intranet.
Treatment Protocol for Vitamin D
Please see full referral guidelines in main body of text
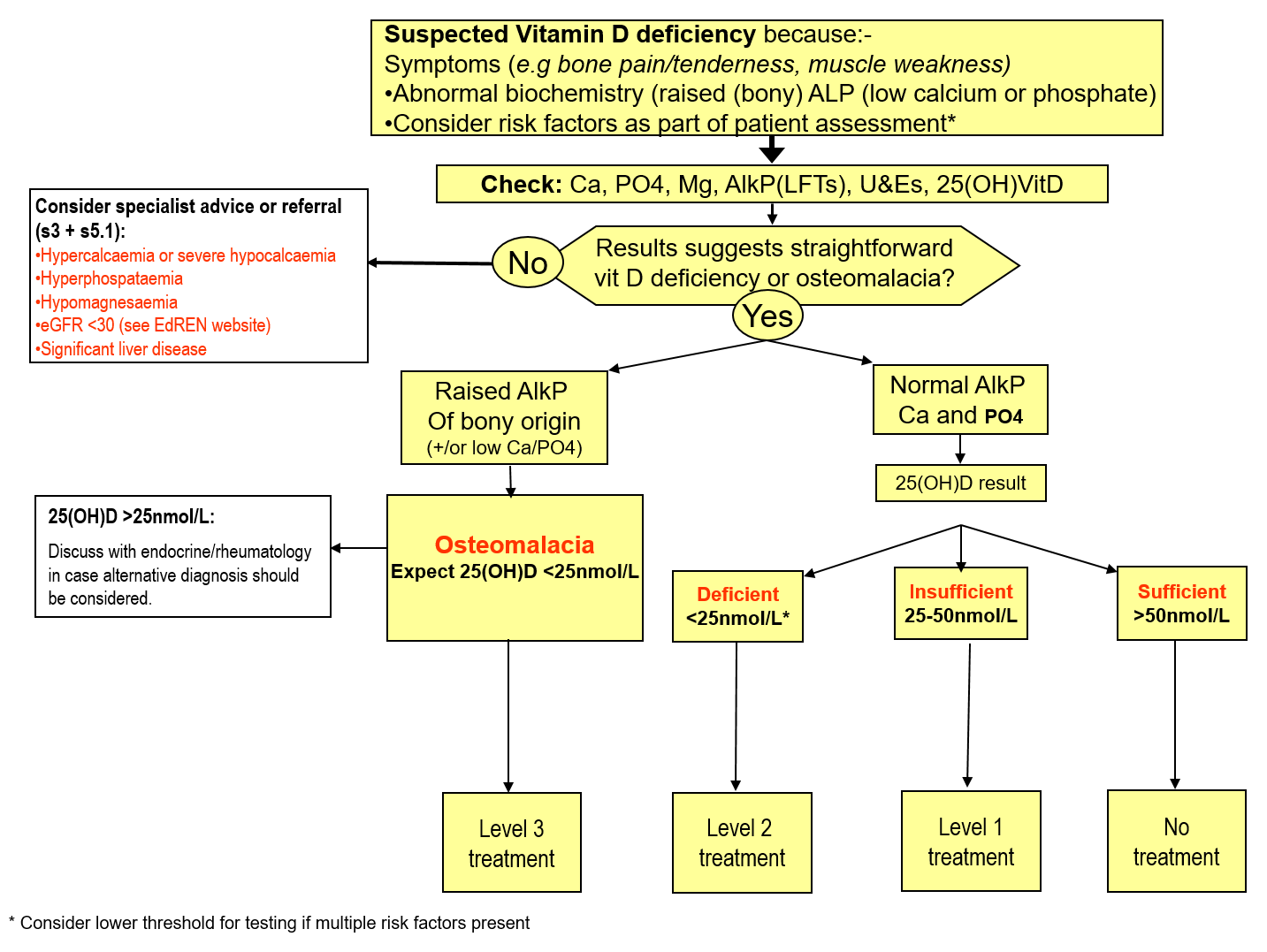
*Level 2 treatment appears adequate.1 In exceptional circumstances (eg 25(OH)D <14 nmol/L AND clearly symptomatic) level 3 treatment might be warranted to rapidly increase vit D level.
Second requests for Vitamin D analysis within 1 year of the first result are not accepted unless special circumstances can be demonstrated.
Table A -Recommended Treatment
Colecalciferol dosing: 10 micrograms are equivalent to 400 units.
The standard (over the counter) CMO recommendation for people over 65 years old and those who lack exposure to sunlight is 10micrograms/400 units of colecalciferol daily.
| Treatment | Indication | Dose of cholecalciferol | Follow up |
| Level 1 | Vitamin D sufficient, but may be at risk of deficiency in future | Lifestyle advice(See section 1.1 and appendix) | Review as clinically indicated. Please also see the CMO guidance about groups needing supplements, and lifestyle advice. |
| Level 2 | Biochemical insufficiency – benefit of treatment uncertain. See section 5.3 prior to commencing. | 800units per day** OR3200units twice weekly**OR 25000units monthly*** | After 6 months, consider whether step down to level 1 treatment appropriate, or if long term treatment required. |
| Level 3 | Osteomalacia– treatment of clear benefit | 25,000units three times weekly for 4 weeks, THEN 25,000units per week for 8 weeks*** | Repeat Ca weeks 2 and 4. Check Ca, AlkP, Phosphate and C/E every 12 weeks. Step down to level 2 treatment when AlkP is normal. |
** Colecalciferol 800units capsules or tablets, 3200units capsules) is approved by SMC and the Lothian Formulary Committee. Please note that 800units is the standard over the counter dose.
*** InVitaD3 25000units/ml solution single use ampoules is a licensed product approved by SMC and the Lothian Formulary Committee. The contents of the ampoule should be emptied directly into the mouth and swallowed or emptied on to a spoon and taken orally. It can also be mixed with a small amount of yoghurt or milk.
****PTH is no longer required for monitoring: it often remains high for a while after Vitamin D stores are replete. The main aim of monitoring is to check that Calcium is not deranged with the higher dose treatments, and the AlkP reflects the response to cholecalciferol. Endocrinology experience is that this is rare unless there is other co-morbidity, or medication likely to affect calcium levels (please see section 3.1 for further detail).
1. Background
There have been large increases in the number of requests received by Glasgow Royal Infirmary clinical chemistry department over the years. This guideline is designed to give practical advice on appropriate testing of vitamin D levels as well as investigation and management of vitamin D deficiency. It has been agreed by senior Lothian endocrine and clinical biochemistry leads, as well as the Primary Care Laboratory Interface Group.
1.1. Sources of Vitamin D
A major source of vitamin D comes from exposure of the skin to sunlight. Skin pigmentation, ageing and use of topical sunscreen all reduce the skin’s production of vitamin D. From October to April, Scotland lies above the latitude that allows exposure to the UV-B wavelengths necessary for vitamin D synthesis2. For a fair-skinned person, 20 to 30 minutes of ‘sub-erythematous’ sunlight exposure at midday on the face and forearms two or three times weekly between April and October are sufficient to achieve healthy vitamin D levels in summer in the UK. However, for individuals with pigmented skin, and to a lesser extent the elderly, exposure time or frequency need to be increased two- to ten-fold to get the same vitamin D synthesis 3.
Dietary sources include oily fish such as trout, salmon, mackerel, herring, sardines, anchovies, pilchards, or fresh tuna which should be consumed 2 to 3 times per week. Cod liver oil and egg yolks also contain vitamin D. Some breakfast cereals are supplemented, and in the UK margarine and infant formula milk have statutory supplementation.
See Resources and links for a table of vitamin D content in selected foods and the patient information leaflet on Vitamin D.
1.2. Vitamin D metabolism and regulation
Vitamin D is made from a precursor (7-dehydrocholesterol) in the skin on exposure to ultraviolet irradiation and is also absorbed from dietary sources. Vitamin D2 (ergocalciferol) is provided by plant sources, and vitamin D3 (colecalciferol) is synthesized in the skin and present in oil rich fish. Both these forms have equivalent biological potencies and are activated efficiently by hydroxylase enzymes in humans. In this guideline, the term vitamin D covers both colecalciferol and ergocalciferol.
Vitamin D undergoes 25-hydroxylation in the liver to 25(OH)D, which has a half life of 2 to 3 weeks. This hydroxylation process is not tightly regulated and therefore the blood levels of 25(OH)D reflects the amount of vitamin D entering the circulation. 25(OH)D is then further hydroxylated in the kidney to the active form 1,25(OH)D, which has a half life of 6 to 8 hours. The renal hydroxylation is tightly regulated; PTH and hypophosphataemia induce the enzyme, while calcium and 1,25(OH)D repress it.
1.3. Definition of insufficient vitamin D
A 25(OH)D level of 25nmol/L is generally recognized as the lower limit required for bone health, based on levels below this being found in patients with osteomalacia and rickets 4, 5. Provocative testing is another method which has been used to quantify optimal vitamin D levels; healthy adults with 25(OH)D levels above 50nmol/L demonstrated no change in PTH levels when challenged with high dose vitamin D 6. There is therefore a feeling that these higher levels of vitamin D should be considered ‘optimal’ and used as a target for treatment.
However, it remains unclear if low levels of vitamin D are causally related to adverse health outcomes 7 and at the present time, evidence that vitamin D supplementation is of benefit in people with ‘biochemical’ deficiency is lacking.
While the clinical significance of cut-offs remains controversial, it has been suggested that people may be categorised into three groups based on their vitamin D levels:
- 25(OH)D <25nmol/l is classified as vitamin D deficiency
- 25(OH)D between 25 and 50nmol/l is classified as vitamin D “insufficiency”
- 25(OH)D between > 50nmol is classified as vitamin D “sufficiency”
Factors increasing the significance of 25(OH)D <50nmol/l include clinical and biochemical features fitting with vitamin D deficiency (sections 3 and 4), and reasons to feel that the vitamin D is usually lower than when measured (eg level was measured during seasonal high point).
2. Actions of Vitamin D
The vitamin D receptor is expressed in most tissues and regulates cellular differentiation and function in many cell types. There are a number of significant associations with low levels of 25(OH)D in observational studies 8, including increased risk of metabolic, neoplastic, and immune disorders (E.g. Type 1 diabetes mellitus and multiple sclerosis). A retrospective, observational study showed a J-shaped association between 25(OH)D levels and mortality 9.
The major physiologic effects of vitamin D involve regulation of intestinal calcium transport. Calcium absorption via the transcellular route and vesicular transport are dependent on 1,25(OH)D. Calcium absorption may also be enhanced by 1,25(OH)D. In the kidney, 1,25(OH)D stimulates calcium reabsorption from the glomerular filtrate.
In the bone 1,25(OH)D has numerous effects, including repression of collagen synthesis and increased osteoclastic bone resorption.
3. Presentation of Vitamin D Deficiency
Vitamin D deficiency in childhood, before epiphyseal closure, results in rickets with skeletal abnormality and muscle weakness. In adulthood the epiphyses are closed, and there is enough mineral in the skeleton to prevent skeletal deformity. The abnormal mineralisation causes decreased bone mineral density, bone and muscle pains, and weakness; this is known as osteomalacia. Bone pain tends to be in the lower spine, pelvis and legs, is often described as dull and aching and is made worse by activity or weight bearing. Muscle weakness also tends to be proximal and may be associated with wasting, hypotonia and discomfort with movement: in more severe cases patients may have a waddling gait, cramps and muscle spasms.
Adults may also be asymptomatic, and diagnosed on biochemistry findings of raised alkaline phosphatase, raised PTH, hypocalcaemia, and hypophosphataemia. Where a significantly raised isolated alkaline phosphatase is found (>200, with no abnormality of GGT or other LFTs), isoenzymes should be requested to confirm bone source prior to investigation for vitamin D deficiency.
3.1. People at risk
Vitamin D deficiency should be considered in those at risk.
A. Those with reduced intake, absorption or synthesis of vitamin D:
- Dark skinned people
- People who cover up (eg Muslim women, people with skin photosensitivity)
- Housebound, institutionalised, and elderly people
- Non-fish eaters (eg vegetarian diets)
- People with fat malabsorption (including those on Orlistat), coeliac disease, small bowel disease and short bowel syndrome
B. Conditions with inadequate vitamin D action requiring specialist management:
- Hypoparathyroidism – most commonly following neck surgery
- Hypomagnesaemia which inhibits PTH release leading to hypoparathyroidism
- People with chronic renal impairment who have a reduced capacity to hydroxylate 25(OH)D into its active form. These people should be discussed with the renal team (see section 5.1)
- Tumour induced osteomalacia.
C. Other causes:
- People with nephrotic syndrome who lose 25(OH)D bound to vitamin D binding protein in the urine.
D. Medications and other co-morbidities – may require assessment of vitamin D status and specialist input:
- Medications including anticonvulsants, rifampicin, drugs to treat HIV as these drugs enhance the catabolism of 1,25(OH)2D 10. These people are at risk of hypocalcaemia, and specialist advice should be considered.
- Hyperparathyroidism causes unregulated hydroxylation of 25(OH)D into the active form, and the resultant hypovitaminosis exacerbate the rise in PTH 11. These patients are hypercalcaemic so the low vitamin D should be managed by a specialist to avoid exacerbating hypercalcaemia.
4. Investigation
Vitamin D deficiency should be considered in people complaining of bone pain, muscle pain or weakness, particularly those who are at risk (see above). It should also be considered in those who have indicative biochemistry performed for other reasons. The characteristic biochemical picture is of raised alkaline phosphatise (isoenzymes indicated only if source is not clear ie bone or liver), raised PTH, hypocalcaemia and hypophosphataemia. The key diagnostic test is decreased serum 25(OH)D.
PTH should not be routinely requested when investigating possible Vitamin D deficiency. It should only be requested if patients have been found to have either hypo or hypercalcaemia.
Patients on vitamin D treatment do not generally require monitoring of vitamin D levels. The possibility of tumour induced osteomalacia should be considered in those with typical features of vitamin D deficiency and hypophosphataemia who do not respond to vitamin D.
Hypoparathyroidism, commonly due to previous surgery but also secondary to low magnesium and other rare causes, can result in hypocalcaemia, hyperphosphataemia with low (or normal) PTH and should be referred to endocrinology for management.
Consideration should be given to the reason for vitamin D deficiency, and coeliac serology should be considered in cases where an underlying cause is not apparent.
25(OH)D should not be routinely measured in those with conditions not linked to bone disease (e.g. cardiovascular disease, malignancy) as there is insufficient evidence to recommend supplementation with vitamin D in this group. Vitamin D testing is not recommended for the standard investigation of ME/CFS unless there are other indications for testing 12.
5. Management and vitamin D replacement considerations
(See treatment protocol)
The general approach is as shown in the algorithm (page 1). If there is any doubt about how a patient should be managed, advice can be requested via an ‘Advice only’ request to endocrinology on SCI Gateway.
5.1 The ‘Who to refer’ section details who might benefit from secondary care input.
5.2. General considerations
Vitamin D therapy is generally safe, even in fairly high doses, hypercalcaemia being the main, but rare, risk. There is varying evidence about times to response, with 2-3 months being quoted by some for the high dose regimes 7.
Normally, as vitamin D levels rise, PTH levels fall and this in turn down regulates 1-alpha-hydroxylase activity. Any condition which causes the activity of 1-alpha-hydroxylase to remain inappropriately high (e.g. hyperparathyroidism) requires caution to avoid hypercalcaemia, and specialist referral is appropriate. Calcium should be monitored to ensure that hypercalcaemia does not occur, particularly in patients with renal dysfunction. Vitamin D levels should not be routinely rechecked.
Hydroxylated vitamin D such as alfa-calcidol (1(OH)D) or calcitriol (1,25(OH)D) can cause hypercalcaemia and are not effective at replenishing vitamin D stores. These preparations may occasionally be used for vitamin D deficiency in secondary care to induce a rapid response but should not be used long term. Their use is largely limited to patients with severe renal impairment leading to reduced renal hydroxylation of 25(OH)D and secondary hyperparathyroidism. These patients should be discussed with a renal physician (see 5.1).
5.3 Treatment Recommendations
All patients should be given lifestyle and dietary advice in the first instance, as described in section 1.1. Please see the patient information leaflet on Vitamin D.
Patients with symptoms of osteomalacia and typical biochemistry require level 3 treatment with high dose vitamin D (Table A).
Patients without these typical features should be assessed with the aid of the treatment algorithm on page 1. There is no good evidence to support intervention in people with vitamin D values between 25-50nmol/L (even with raised PTH).
Following a course of treatment, patients should be reassessed to ensure that the treatment has had the desired effect, and to consider whether long term vitamin D replacement is required. This should be done by clinically reassessing for signs and symptoms of vitamin D deficiency. Also, any biochemical abnormality should be repeated following a course of treatment to ensure normalisation (eg raised AlkP). A repeat vitamin D level is generally not required, unless it is felt that the initial test did not reflect current vitamin D levels due to mitigating factors already discussed. Serial PTH testing is not helpful and should be avoided.
Long term treatment should be considered in those with ongoing, irreversible risk factors. If diet and sunlight exposure cannot be improved in this group, then low dose treatment with 800units of vitamin D daily may be required to prevent repeated episodes of osteomalacia. Evidence to support this is lacking.
5.4. Supplementation with low dose vitamin D
Recent endocrine society clinical practice guidelines 4 suggest that adults aged 19 – 70 years require at least 600units/day of vitamin D to maximise bone health and muscle function. Similarly, adults over 70 years require at least 800units/day of vitamin D. There is evidence that supplementing elderly patients with vitamin D reduces non-vertebral fracture risk (>400units per day) as well as falls risk (>700units per day and 25(OH)D levels >60nmol/L) 13. However, some other trials show no benefit on fracture and falls with vitamin D supplementation in the elderly 8, 14
Calcium and vitamin D supplementation is recommended for certain elderly patients classed as being at risk of falls as part of the falls risk strategy (see the NHS Lothian Falls Policy). The Chief Medical Officers of the UK have also recommended routine supplementation with 400units of vitamin D per day in those over 65years, and those not exposed to much sun.
Excessive vitamin D supplementation can be harmful and lead to hypercalcaemia. Some OTC supplements are very high dose e.g. up to 10,000 units. Scottish government guidance is that a dose of 400 units is sufficient for most people and also recommends that adults and children over 11 should avoid daily high dose vitamin D supplements containing more than 4000 units due to the risk of harm
5.5. Non musculoskeletal conditions and screening
There is observational evidence that low vitamin D levels are associated with increased mortality, cardiovascular mortality, type 2 diabetes, cancer and multiple sclerosis. There is no evidence to support treatment with vitamin D for these conditions 15, nor for screening for vitamin D deficiency in the general population 4, 13
Original author: B Inkster, May 2013. Reviewed Feb 2022 – Ganesan Arunagirinathan, Catriona Morton, Nicola Zammitt.
C.M. & N.Z. 20-02-23
Who to refer:
Secondary care referral should be considered for:
- Patients with severe pain or weakness
- Emergency (same day) treatment for those with severe hypocalcaemia (Ca2+ usually <1.9mmol/l with muscle twitches, convulsions, Chvostek’s sign, Trousseau’s sign, carpal spasm, papilloedema, prolonged QT interval on ECG) or hypomagnesaemia, who may need iv replacement
- Any patient who does not respond to vitamin D replacement as expected
- Patients with renal failure (eGFR <30) who may need ‘activated’ vitamin D, or lower doses of vitamin D, should be discussed with the renal team; referral Patients with GI disorders who may require high doses or parenteral administration of vitamin D should be referred to GI
- Patients with probable primary hyperparathyroidism (raised Ca and normal or high PTH) should be referred to endocrinology. This may become uncovered by vitamin D treatment causing Ca2+ levels to rise.
- Patients with hypoparathyroidism should be referred to endocrinology (Low PTH, low Ca, low/normal 25(OH)D, e.g. patients with history of neck surgery).
- Patients with osteoporosis should be managed as per osteoporosis guidance.
- guidelines can be found here: http://www.edren.org/pages/gpinfo/when-to-refer-to-the-renal-unit.php For advice: rierenaladvice@luht.scot.nhs.uk
- Patients with nephrotic syndrome should also be discussed with the renal team.
How to refer:
Please refer via SCI Gateway to Endocrinology: please note that there is an advice-only option.
Vitamin D and You in multiple other languages
Appendix 1: Selected food sources of vitamin D 16
| Food | Vitamin D per serving (International Units) |
| Cod liver oil, 1 tablespoon | 1,360 |
| Salmon, cooked, 3 ounces | 570 |
| Mackerel, cooked, 3.5 ounces | 500 |
| Tuna fish, canned in water, 3 ounces | 40 |
| Sardines, canned in oil, drained, 2 sardines | 46 |
| Milk, non fat, reduced fat, and whole, vitamin D-fortified, 1 cup (US) | 98 |
| Milk, non fat, reduced fat and whole, 1 cup (UK; non fortified) 1 cup | 10 |
| Mushrooms, white, raw, sliced, exposed to UV light, ½ cup | 366 |
| Mushrooms, portabella, raw, diced, ½ cup | 4 |
| Margarine, fortified, 1 tablespoon | 60 |
| Ready-to-eat cereal, fortified with 10% of the DV for vitamin D, 1 serving (more heavily fortified cereals might provide more of the DV) | 80 |
| Egg, 1 large scrambled (vitamin D is found in yolk) | 44 |
| Liver, beef, braised, 3 ounces | 42 |
| Chicken breast, roasted, 3 ounces | 4 |
| Cheese, cheddar, 1.5 ounce | 17 |
Appendix 2: References
1. Ralston SH, Binkley N, Boonen S et al. Randomised trial of alendronate plus vitamin D3 versus standard care in osteoporotic postmenopausal women with vitamin D insufficiency. Calcif Tissue Int 2011;88:485-494.
2. Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab 1988; 67:373-8.
3. Ross W, Pearce S, Skinner J et al. Vitamin D guidelines: NHS Newcastle North Tyneside and Northumberland.
4. Holick, MF, Binkley NC, Bischoff-Ferrari, HA et al. Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J Clin Endocrin Metab 2011;96(7)
5. Pearce SHS, Cheetham TD. Diagnosis and management of vitamin D deficiency. BMJ 2010;340:142-7.
6. Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet 1998;351:805-6
7. Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JPA. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ 2014; 348:g2035
8. Rosen CJ. Vitamin D Insufficiency. N Engl J Med 2011;364:248-54
9. Durup D, Jorgensen HL, Christensen J et al. A reverse J-shaped association of all cause mortality with serum 25-hydroxyvitamin D in general practice, the CopD study. J Clin Endocrinol Metab 2012;97(8):1-9
10. Zhou C, Assem M, Tay JC et al. Steroid and xenobiotic receptor and vitamin D receptor crosstalk mediates CYP24 expression and drug-induced osteomalacia. J Clin Invest 2006;116:1703-12
11. Grey A, Lucas J, Horne A et al. Vitamin D repletion in patients with primary hyperparathyroidism and coexistent vitamin D insufficiency J Clin. Endocrinol. Metabolism 2005; 90:2122-6
12. Myalgic encephalomyelitis (or encephalopathy)/chronic fatigue syndrome: diagnosis and management. Nice Guideline 206. Oct 2021
13. Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc 2011; 86(1): 50-60
14. Aloia, JF. The 2011 report on dietary reference intake for vitamin D: Where do we go from here? J Clin Endocrin Metab. 2011;96(10):2987-96
15. Sattar N, Welsh P, Panarelli M et al. Increasing requests for vitamin D measurement: costly, confusing and without credibility. Lancet 2012;379:95-6
16. National institute of health office of dietary supplements ‘Heath Professionals Factsheet on Vit D-Dec 2021













